Ernest Rutherford his work presentation. Presentation on the topic "Ernest Rutherford". What do atoms and quanta have in common?
slide 1
 slide 2
slide 2
 slide 3
slide 3
 slide 4
slide 4
 slide 5
slide 5
 slide 6
slide 6
 Slide 7
Slide 7
 Slide 8
Slide 8
 Slide 9
Slide 9
 Slide 10
Slide 10
The presentation on the topic "Ernest Rutherford" can be downloaded absolutely free of charge on our website. Project subject: Physics. Colorful slides and illustrations will help you keep your classmates or audience interested. To view the content, use the player, or if you want to download the report, click on the appropriate text under the player. The presentation contains 10 slide(s).
Presentation slides

slide 1
Ernest Rutherford

slide 2
Ernest Rutherford is considered the greatest experimental physicist of the twentieth century. He is the central figure in our knowledge of radioactivity, and also the man who laid the foundation for nuclear physics. In addition to their great theoretical significance, his discoveries have received a wide range of applications, including: nuclear weapons, nuclear power plants, radioactive calculations and radiation research. The impact of Rutherford's work on the world is enormous. It continues to grow and is likely to increase further in the future.

slide 3

slide 4
In New Zealand in 1889 he entered Canterbury College and by the age of twenty-three received three degrees (Bachelor of Arts, Bachelor of Science, Master of Arts). The following year he was awarded the right to study at the University of Cambridge in England, where he spent three years as a research student under J. J. Thomson, one of the leading scientists of the day. At twenty-seven, Rutherford became a professor of physics at McGill University in Canada. He worked there for nine years and returned to England in 1907 to head the physics department at the University of Manchester. In 1919, Rutherford returned to Cambridge, this time as director of the Cavendish Laboratory, and remained in this post for the rest of his life.

slide 5
One of Rutherford's first discoveries was that the radioactive radiation from uranium consists of two different components, which the scientist called alpha and beta rays. Later, he demonstrated the nature of each component (they are composed of fast moving particles) and showed that there is also a third component, which he called gamma rays.

slide 6
But Rutherford found that some alpha particles passing through gold foil were deflected very strongly. In fact, some even fly back! Feeling that there was something important behind this, the scientist carefully counted the number of particles that flew in each direction. Then, through complex but quite convincing mathematical analysis, he showed the only way in which the results of the experiments could be explained: the gold atom consisted almost entirely of empty space, and almost all of the atomic mass was concentrated in the center, in the small "nucleus" of the atom!

Slide 8

Slide 9

He was one of 12 children of James Rutherford, a wheelwright and construction worker, of Scottish descent, and Martha (Thompson) Rutherford, an English schoolteacher. First R. attended primary and secondary local schools, and then became a fellow of Nelson College, a private high school, where he proved himself a talented student, especially in mathematics. Due to academic excellence R. received another scholarship, which allowed him to enroll in Canterbury College in Christchurch, one of the largest cities in New Zealand.
In college, R. was greatly influenced by his teachers: who taught physics and chemistry, E.U. Bickerton and mathematician J. Cook. After in 1892, Mr.. R. was awarded a Bachelor of Arts degree, he remained at Canterbury College and continued his studies thanks to a scholarship in mathematics.

The following year, he became a master of arts, having passed the exams in mathematics and physics with the best of all. His master's work concerned the detection of high-frequency radio waves. In order to study this phenomenon, he constructed a wireless radio receiver and used it to receive signals transmitted by colleagues from a distance of half a mile.

In 1894, Mr.. R. was awarded a bachelor's degree in natural sciences. There was a tradition at Canterbury College that any student who completed an M.A. and remained at the college was required to undertake further research and receive a B.Sc.

In Cambridge, R. worked under the guidance of the English physicist J.J. Thomson. Their collaboration was crowned with significant results, including Thomson's discovery of the electron, an atomic particle that carries a negative electrical charge. Based on their research, Thomson and R. suggested that when X-rays pass through a gas, they destroy the atoms of this gas, releasing the same number of positively and negatively charged particles. They called these particles ions.




In 1911 he proposed a new model of the atom. According to his theory, which has become generally accepted today, positively charged particles are concentrated in the heavy center of the atom, and negatively charged particles (electrons) are in orbit of the nucleus, at a fairly large distance from it.

In 1919, Mr.. R. moved to the University of Cambridge, becoming Thomson's successor as professor of experimental physics and director of the Cavendish Laboratory, and in 1921 took up the position of professor of natural sciences at the Royal Institution in London. In 1930, Mr.. R. was appointed chairman of the government advisory board of the Office of Scientific and Industrial Research. Being at the top of his career, the scientist attracted many talented young physicists to work in his laboratory in Cambridge.

In 1900, during a brief trip to New Zealand, R. married Mary Newton, who bore him a daughter. Almost to the end of his life, he was distinguished by good health and died in Cambridge in 1937 after a short illness. R. buried in Westminster Abbey near the graves of Isaac Newton and Charles Darwin.


Sitnikov Arseniy
This presentation tells about the life of Rutherford, his contribution to the scientific and social life of England.
Download:
Preview:
To use the preview of presentations, create a Google account (account) and sign in: https://accounts.google.com
Slides captions:
Life in science Research project of students of the Saratov Secondary School No. 103 Sitnikova Arkadia, Smirnova Egora
“All science is either physics or stamp collecting” This work is dedicated to the English physicist who was awarded the Nobel Prize in 1908, the founder of nuclear physics, the scientist who did a lot to establish the physical picture of the world - Ernest Rutherford.
Stages of life of an English physicist - E. Rutherford, E. Rutherford was born on 30.08. 1871 in New Zealand, in a large family; Studied excellently at Canterbury College of the Humanities, University of New Zealand; 1892 - received a Bachelor of Arts degree; 1894 - received a bachelor's degree in natural sciences; 1895 - Master of Arts, having passed the exams in mathematics and physics best of all .; 1895 - as the best student, he was sent to England to the Cavendish Laboratory of Cambridge under the direction of J. Thompson; Library in Cambridge
Stages of life - the "father" of nuclear physics E. Rutherford 1897 - Professor at McGill University in Montreal (Canada) 1903 - Member of the Royal Society of London; 1907 - England, Victoria University of Manchester; 1919 - professor at the University of Cambridge and director of the Cavendish Laboratory; 1921 Professor of Natural Sciences at the Royal Institution in London; 1930 - Chairman of the Government Advisory Board of the Office of Scientific and Industrial Research; On October 19, 1937, he died in Cambridge and was buried in Westminster Abbey and the Arch at the entrance to the Cavendish Laboratory
Models of atoms Thomson's model of the atom - "Pudding with raisins". Rutherford's model of the atom is planetary In the center of the atom, like the Sun in the solar system, there is a nucleus, in which, despite its relatively small size, the entire mass of the atom is concentrated. And around it, like planets moving around the Sun, electrons revolve.
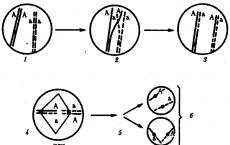
Rutherford's main experiment Bombardment of a thin plate of gold with α-particles K - lead container with a radioactive substance, E - screen coated with zinc sulfide, F - gold foil, M - microscope 1-atom of gold 2-α-particles
The results of Rutherford's experiments 1. Most of the particles pass through the atoms of matter. not dissipating (as through "emptiness"); 2. with an increase in the scattering angle, the number of particles deviated from the original direction decreases sharply; 3. there are separate particles thrown back by atoms against their initial motion (like a ball from a wall).
Colleagues and students of E. Rutherford In his work and life, E. Rutherford met with many scientists, physicists, chemists, future Nobel Prize winners: Joseph John Thomson Petr Leonidovich Kapitsa Maria Sklodowska-Curie Henry Moseley James Chadwick Enrico Fermi
Colleagues and students of Rutherford Niels Bohr Otto Hahn Henri Becquerel Hans Geiger Ernest Marsden Georgy Antonovich Gamov
Society recognition 1914 Nobility 1923 President of the British Association 1925 President of the Royal Society 1931 Baron Lord Rutherford of Nelson 1931 Peerage 1933 President of the Academic Council for Relief for German Fugitives
Recognition of the scientific merits of E. Rutherford 1904 - Rumford Medal of the Royal Society of London; 1908 - Nobel Prize in Chemistry "for his research in the field of the decay of elements in the chemistry of radioactive substances"; 1922 - Copley Medal of the Royal Society of London; 1926 - British Order of Merit; Corresponding Member of the Royal Society of Canada, American Physical Society, British Association for the Advancement of Progress, Royal Society of Göttingen, New Zealand Philosophical Institute, American Philosophical Society. St. Louis Academy of Sciences, Royal Society of London
The Meaning of Ernest Rutherford's Life "Rutherford never made an enemy or lost a friend in his whole life." (Friend's statement) "That I am Lord Rutherford is more your merit than mine. Your son Ernest." (From a letter to my mother) “I have dealt with many different transformations with different periods, but the fastest of all was my own transformation in one moment from a physicist to a chemist” (from a speech at the Nobel Prize) All the sciences of Nature are divided into physics and stamp collecting (from E. Rutherford's speech)
Significance of the scientific life of Ernest Rutherford Three stages of recognition of scientific truth: the first - "this is absurd", the second - "there is something in this", the third - "it is well known" (E. Rutherford's aphorism) ... each outstanding researcher contributes his name in the history of science not only by his own discoveries, but also by those discoveries to which he encourages others. (M. Planck) "Rutherford's life is an almost continuous chain of brilliant discoveries. (Academician Yu.B. Khariton) Nuclear physics "was actually his area scientific activity” (Patrick Blackett)
Conclusions: 1. Studying the biographies of scientists is an interesting business; 2.Create a video library about great physicists in the physics classroom; 3. Make up a conversation-excursion about E. Rutherford, conduct a conversation in the lower and middle grades; 4. Write to the "School newspaper" about E. Rutherford under the heading "They write about them, they say ..."; 5. Speak at the NPC at the school in plenary session. Thanks for attention
References and Internet resources Internet resources http://ru.wikipedia.org/wiki/%C0%F2%EE%EC http://www.edu.delfa.net/Interest/biography/biblio.htm http:// textik.ru/citations/topic/science/? fiz.1september.ru›2006/21/12.htm http:// class-izika.narod.ru/9_35.htm http:// fizika.ayp.ru/9/9_1.html http://www.newreferat .com/ref-12715-1.html Literary sources Big encyclopedic dictionary. Physics / ch. ed. A. M. Prokhorov. - M.: Great Russian Encyclopedia, 1998. Chemical Encyclopedic Dictionary / ch. ed. I.L. Knunyants. - M.: Sov. encyclopedia, 1983. F. Fedorov. "The Chain Reaction of an Idea", ed. "Knowledge", M., 1975 T.I. Trofimova. "Course of Physics", ed. "Higher School", M., 1999 "Course of General Physics", G.A. Zisman, O.M.Todes, ed. "Edelweiss", Kyiv, 1994
Ernest Rutherford
Born in New Zealand. The son of a cattle farmer. From 1895 he worked at the Cavendish Laboratory. He was the first "overseas doctoral student".
Participated in work on the study of cathode and X-rays under the direction of J. J. Thomson. Developed the "Magnetic Electromagnetic Wave Detector".
Since 1898 he has been engaged in the study of radioactivity. Established the charge and mass of particles. He proved (together with F. Soddy) that an atom of another element appears in the process of radioactive decay. Established the law of radioactive decay.
In 1898 - 1907. worked in Montreal (Canada), in 1907 - 1919. - University of Manchester (UK). 1919 - 1937 director of the Cavendish Laboratory.

Particle Research
Scattering of -particles by a plate of mica

1909 Rutherford, Marsden, Geiger.
It has been found that one of about 8000 particles is scattered through an angle close to 180°.
Hans Geiger

Scattering of particles by gold foil
Rutherford wrote: “It is as incredible as if you fired a 15-inch projectile at a piece of thin paper, and the projectile came back to you and struck.”

Scattering of particles by gold foil
The probability of such a "reversal" of the -particle is much less than 1/8000. It is practically equal to zero.

Scattering of particles by gold foil
the only
A plausible explanation for the large-angle scattering of -particles was the presence of a massive, albeit very small, positively charged body inside the atom.
In late 1910, Rutherford told Geiger, "I know how
looks like an atom.
At the very beginning of the article in "Phylosophical Magazine" it is written: "It is still premature to consider the question of the stability of the atom"

And yet they spin?
Reasons why a planetary atom cannot exist:
- electrons move along closed paths, therefore, they move with acceleration;
- charged particles moving with acceleration must radiate EMW and therefore lose energy, and hence speed;
- as a result, the electron must fall on the nucleus.
6.4. rule
Bohr-Sommerfeld quantization.

What do the atom and quanta have in common?
Niels Bohr (1885 - 1962)
1. In order to explain how Rutherford's planetary atom can exist, Bohr put forward a bold assumption that the property of quantization, that is, discrete change, is inherent not only in light quanta - photons, but has a deeper meaning.
2. He drew attention to the fact that Planck's constant h has a dimension (in the SI system, which did not exist then, J s), coinciding with the dimension of a physical quantity called action.
3. Bohr suggested that all physical quantities should change in such a way that in the course of any processes the action should be equal to an integer number of Planck's constants h.
4. Based on the assumption of quantization (jump change) of the action, Bohr suggested that when an electron moves in an orbit around a positively charged nucleus, the condition
that is, the action of the electron is quantized.
Bohr-Sommerfeld quantization rule
According to Planck, the emission of light occurs in portions - quanta, the energy
which is equal to h
E h .
The change in the energy of a standing wave that occurs in a cavity around a blackbody is also equal to E h h .
Standing waves can be viewed as linear harmonic oscillators. According to Planck, of all the possible states of a linear harmonic oscillator, only those are realized whose energy
slide 1
slide 2

slide 3
 In 1911, Rutherford experimentally tested Thomson's model of the atom. Passing a beam of α-particles through a thin gold foil, Ernest Rutherford found that some part of the particles deviated by a fairly significant angle from their original direction, and a small part of the α-particles was reflected from the foil. But according to the Thomson model of the atom, these α-particles, when interacting with foil atoms, should deviate through small angles, on the order of 2˚. The results of the experiment surprised Rutherford so much that he exclaimed: "... implausible, just as if you fired a fifteen-pound projectile at tissue paper, and the projectile would bounce back and kill you yourself." Rutherford showed that Thomson's model was in conflict with his experiments. Rutherford's main experiment
In 1911, Rutherford experimentally tested Thomson's model of the atom. Passing a beam of α-particles through a thin gold foil, Ernest Rutherford found that some part of the particles deviated by a fairly significant angle from their original direction, and a small part of the α-particles was reflected from the foil. But according to the Thomson model of the atom, these α-particles, when interacting with foil atoms, should deviate through small angles, on the order of 2˚. The results of the experiment surprised Rutherford so much that he exclaimed: "... implausible, just as if you fired a fifteen-pound projectile at tissue paper, and the projectile would bounce back and kill you yourself." Rutherford showed that Thomson's model was in conflict with his experiments. Rutherford's main experiment
slide 4
 E. Rutherford was born on 30.08. 1871 in New Zealand, in a large family; Studied excellently at Canterbury College of the Humanities, University of New Zealand; 1892 - received a Bachelor of Arts degree; 1894 - received a bachelor's degree in natural sciences; 1895 - Master of Arts, having passed the exams in mathematics and physics best of all .; 1895 - as the best student, he was sent to England to the Cavendish Laboratory of Cambridge under the direction of J. Thompson; Stages of life - the "father" of nuclear physics E. Rutherford Library in Cambridge
E. Rutherford was born on 30.08. 1871 in New Zealand, in a large family; Studied excellently at Canterbury College of the Humanities, University of New Zealand; 1892 - received a Bachelor of Arts degree; 1894 - received a bachelor's degree in natural sciences; 1895 - Master of Arts, having passed the exams in mathematics and physics best of all .; 1895 - as the best student, he was sent to England to the Cavendish Laboratory of Cambridge under the direction of J. Thompson; Stages of life - the "father" of nuclear physics E. Rutherford Library in Cambridge
slide 5

slide 6
 Rutherford was born in New Zealand in the small village of Spring Grove (eng. Spring Grove), located in the north of the South Island near the city of Nelson, in the family of a farmer who grew flax. Father - James Rutherford, immigrated from Perth (Scotland). Mother - Martha Thompson, originally from Hornchurch, Essex, England. At this time, other Scots emigrated to Quebec (Canada), but the Rutherford family was unlucky and the government provided a free steamboat ticket to New Zealand, and not to Canada. Ernest was the fourth child in a family of twelve children. He had an amazing memory, good health and strength. Sculpture of a young Ernest Rutherford. Memorial in New Zealand Biography
Rutherford was born in New Zealand in the small village of Spring Grove (eng. Spring Grove), located in the north of the South Island near the city of Nelson, in the family of a farmer who grew flax. Father - James Rutherford, immigrated from Perth (Scotland). Mother - Martha Thompson, originally from Hornchurch, Essex, England. At this time, other Scots emigrated to Quebec (Canada), but the Rutherford family was unlucky and the government provided a free steamboat ticket to New Zealand, and not to Canada. Ernest was the fourth child in a family of twelve children. He had an amazing memory, good health and strength. Sculpture of a young Ernest Rutherford. Memorial in New Zealand Biography
Slide 7
 Rutherford's main experiment Bombardment of a thin plate of gold with α-particles K - lead container with a radioactive substance, E - screen coated with zinc sulfide, F - gold foil, M - microscope 1-atom of gold 2-α-particles
Rutherford's main experiment Bombardment of a thin plate of gold with α-particles K - lead container with a radioactive substance, E - screen coated with zinc sulfide, F - gold foil, M - microscope 1-atom of gold 2-α-particles
Slide 8
 Model of the atom In the center of the atom, like the Sun in the solar system, there is a nucleus, in which, despite its relatively small size, the entire mass of the atom is concentrated. And around it, like planets moving around the Sun, electrons revolve.
Model of the atom In the center of the atom, like the Sun in the solar system, there is a nucleus, in which, despite its relatively small size, the entire mass of the atom is concentrated. And around it, like planets moving around the Sun, electrons revolve.
Slide 9
 Colleagues and students of E. Rutherford In his work and life, E. Rutherford met with many scientists, physicists, chemists, future Nobel Prize winners: Joseph John Thomson Petr Leonidovich Kapitsa Maria Sklodowska-Curie Henry Moseley James Chadwick Enrico Fermi
Colleagues and students of E. Rutherford In his work and life, E. Rutherford met with many scientists, physicists, chemists, future Nobel Prize winners: Joseph John Thomson Petr Leonidovich Kapitsa Maria Sklodowska-Curie Henry Moseley James Chadwick Enrico Fermi
slide 10
 Society recognition 1914 - received a title of nobility and becomes "Sir Ernst" 1923 - President of the British Association 1925 - President of the Royal Society 1931 - Baron, Lord Rutherford of Nelson 1931 - Peerage 1933 - President of the Academic Council for Relief, Fugitives from Germany
Society recognition 1914 - received a title of nobility and becomes "Sir Ernst" 1923 - President of the British Association 1925 - President of the Royal Society 1931 - Baron, Lord Rutherford of Nelson 1931 - Peerage 1933 - President of the Academic Council for Relief, Fugitives from Germany
slide 11
 Rutherford's personality constantly amazed everyone who met him. He was a big man with a loud voice, boundless energy, and a marked lack of modesty. When colleagues noted Rutherford's supernatural ability to always be "on the crest of a wave" of scientific research, he immediately answered: "Why not? After all, I caused the wave, didn't I?" Few scientists would object to this assertion. Rutherford's personality
Rutherford's personality constantly amazed everyone who met him. He was a big man with a loud voice, boundless energy, and a marked lack of modesty. When colleagues noted Rutherford's supernatural ability to always be "on the crest of a wave" of scientific research, he immediately answered: "Why not? After all, I caused the wave, didn't I?" Few scientists would object to this assertion. Rutherford's personality
slide 12
 Interesting facts For the good nature of the students nicknamed Rutherford the Crocodile. In 1931, Krokodil secured £15,000 for the construction and equipment of a special laboratory building for Kapitsa. In February 1933, the laboratory was inaugurated in Cambridge. On the end wall of a 2-storey building, a huge crocodile was carved in stone, covering the entire wall. It was commissioned by Kapitza and made by the famous sculptor Eric Gill. Rutherford himself explained that it was him. The front door was opened with a gilded key in the shape of a crocodile. E. Rutherford, who discovered the nucleus of the atom, spoke negatively about the prospects for nuclear energy: “Everyone who hopes that the transformation of atomic nuclei will become a source of energy confesses nonsense.” When Pyotr Kapitsa came to work in Cambridge to Rutherford, he told him that the laboratory staff completed. Then Kapitsa asked: - What is the allowable error you allow in the experiments? - Usually about 3% - And how many people work in the laboratory? - 30 - Then 1 person is about 3% of 30 Rutherford laughed and accepted Kapitsa as a "permissible error." Having received the news in 1908 that he had been awarded the Nobel Prize in Chemistry, Rutherford declared: “All science is either physics or stamp collecting.” Ernest Rutherford’s great-grandson, Michael Rutherford, is known for his participation in the program rock band Genesis and his band Mike & the Mechanics.
Interesting facts For the good nature of the students nicknamed Rutherford the Crocodile. In 1931, Krokodil secured £15,000 for the construction and equipment of a special laboratory building for Kapitsa. In February 1933, the laboratory was inaugurated in Cambridge. On the end wall of a 2-storey building, a huge crocodile was carved in stone, covering the entire wall. It was commissioned by Kapitza and made by the famous sculptor Eric Gill. Rutherford himself explained that it was him. The front door was opened with a gilded key in the shape of a crocodile. E. Rutherford, who discovered the nucleus of the atom, spoke negatively about the prospects for nuclear energy: “Everyone who hopes that the transformation of atomic nuclei will become a source of energy confesses nonsense.” When Pyotr Kapitsa came to work in Cambridge to Rutherford, he told him that the laboratory staff completed. Then Kapitsa asked: - What is the allowable error you allow in the experiments? - Usually about 3% - And how many people work in the laboratory? - 30 - Then 1 person is about 3% of 30 Rutherford laughed and accepted Kapitsa as a "permissible error." Having received the news in 1908 that he had been awarded the Nobel Prize in Chemistry, Rutherford declared: “All science is either physics or stamp collecting.” Ernest Rutherford’s great-grandson, Michael Rutherford, is known for his participation in the program rock band Genesis and his band Mike & the Mechanics.
slide 13
 List of Internet resources http://ru.wikipedia.org/wiki/%C0%F2%EE%EC http://www.edu.delfa.net/Interest/biography/biblio.htm http://textik.ru/ citations/topic/science/? fiz.1september.ru›2006/21/12.htm http://class-izika.narod.ru/9_35.htm http://fizika.ayp.ru/9/9_1.html http://www.newreferat .com/ref-12715-1.html
List of Internet resources http://ru.wikipedia.org/wiki/%C0%F2%EE%EC http://www.edu.delfa.net/Interest/biography/biblio.htm http://textik.ru/ citations/topic/science/? fiz.1september.ru›2006/21/12.htm http://class-izika.narod.ru/9_35.htm http://fizika.ayp.ru/9/9_1.html http://www.newreferat .com/ref-12715-1.html
slide 14